Consumer Products & Advertising — Increasing Focus on Health Claims for Cosmetics and Personal Care Products
Publication | 01.19.16
The aging U.S. population creates an attractive and lucrative marketing opportunity. The Federal Trade Commission (FTC) and the Food and Drug Administration (FDA), as well as state regulators and private litigants, will increasingly scrutinize products claiming to slow or ease the effects of aging or to address specific age-related conditions.

“The FTC and FDA have successfully challenged health claims for pharmaceuticals, devices and apps, food, and dietary supplements, and now it seems likely that cosmetics and personal care products are next,” says David Ervin, a partner in Crowell & Moring’s Advertising & Product Risk Management Group. As Ervin points out, “Any products that claim to reduce wrinkles, help you appear younger, or feel better—especially any that tout a scientific or clinical basis for their effectiveness—are likely to be of great interest to regulators.”
Products claiming to provide scientific solutions for age-related conditions will be in the crosshairs, agrees Peter Miller, senior counsel at Crowell & Moring, who served as an FTC attorney for more than a decade. In 2014, the FTC settled an enforcement action arising from claims by cosmetics company L’Oréal that two of its skincare products provided anti-aging benefits by targeting users’ genes. In 2015, the FTC brought enforcement actions against three marketers that claimed that the catalase enzyme in their products—“Get Away Gray,” “Go Away Gray” and “Grey Defence” dietary supplements and “Go Away Gray” daily anti-gray shampoo and conditioner—prevented or reversed the formation of gray hair.
A landmark federal appellate ruling in early 2015 affirmed both the breadth of the FTC’s advertising enforcement jurisdiction and its interpretation of health and disease claims. The U.S. Court of Appeals for the D.C. Circuit, agreeing almost completely with the FTC, affirmed that POM Wonderful made unwarranted health and disease-prevention claims when advertising its pomegranate juice products.
The 2015 POM Wonderful decision also provided a victory for marketers regarding the substantiation required for health and disease claims. Rejecting the FTC’s assertion that two randomized human clinical trials (RCTs) were required to substantiate disease claims—a requirement common to many FTC orders—the D.C. Circuit ruled that one RCT would generally be sufficient. With RCTs costing $100,000 to $150,000 and more, that was especially good news for marketers making health and disease-related claims, Ervin says.
Miller notes that marketers of cosmetics and personal care products don’t just have to fear federal regulators; they are increasingly being taken to task by competitors, watchdog groups, class action litigants, and state attorneys general as well. “It really has become a multi-risk environment,” he says. “Knowing how to safely thread your way through it is extremely important.”
One way marketers can protect themselves is through effective vendor management. “You’re responsible for what ends up in the package and what you say about it,” Miller says. “You need to know the sources, the ingredients, the manufacturer, and the amount and type of substantiation that has been done.”
BUILDING PRIVACY AND SECURITY INTO THE IoT
The FTC kicked off 2015 with a report providing privacy and security guidance regarding the growing variety of Internet-connected devices and sensors known as the Internet of Things (IoT). The FTC followed up by creating the new Office of Technology Research and Investigation (OTECH), which will tackle technology issues such as “privacy, data security, connected cars, smart homes, algorithmic transparency, emerging payment methods, big data, and the Internet of Things.” In November, the FTC conducted a Cross-Device Tracking Workshop, and in January 2016, it will hold its first-ever PrivacyCon, a conference at which industry leaders will discuss the latest research and trends in consumer privacy and data security.
Miller says that non-tech companies new to IoT are at the greatest risk for running afoul of regulators, including the FTC, in 2016. For example, alcoholic beverage marketers have discussed using “smart bottles” to increase interactions with and to collect information about consumers. “These are companies that have never collected large amounts of data directly from their customers,” says Ervin. “And there are a host of regulatory and compliance issues that have to be considered.”
The FTC’s IoT report includes specific privacy and security recommendations, including, for example, that companies consider data minimization—or voluntarily limiting the amount of data collected—and take steps to de-identify the data they do collect. “The point is that privacy and security can’t just be afterthoughts,” says Ervin. “They have to be built into the plan from day one.”
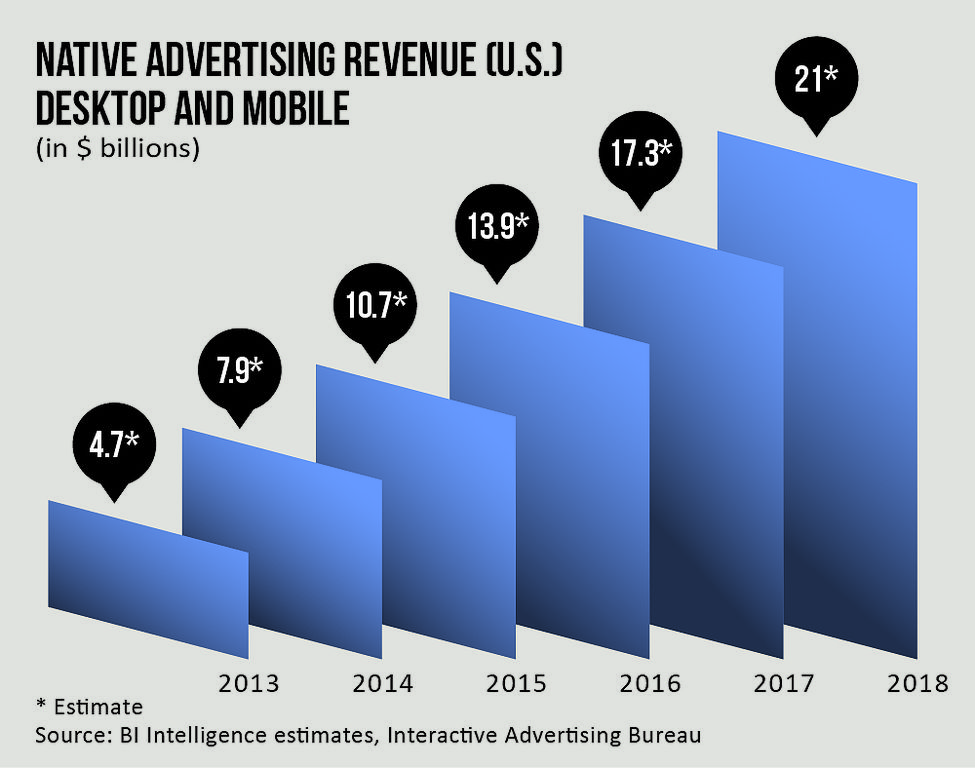
[Despite concerns about possible regulations, native advertising has been a boon for publishers, accounting for billions in revenue, with BI Intelligence and the Interactive Advertising Bureau estimating a nearly fivefold increase in as many years.]
Advertising: Unblurring the Lines
On December 22, 2015, the Federal Trade Commission (FTC) issued its highly anticipated Enforcement Policy Statement on Deceptively Formatted Advertisements, which addresses native advertising, a popular form of advertiser-sponsored content that merges marketing with non-commercial content. The FTC also released Native Advertising: A Guide for Businesses to identify practices that prevent deceptive use of native advertising.
According to Ervin, some of the FTC’s guidance—such as placing disclosures on sponsored images and graphics, requiring disclosures to survive republication, and declaring company logos and names alone to be insufficient disclosures—sets up a potential battle with publishers, which see native advertising as a way to make up for lost revenue resulting from flagging print publications, nonperforming online banner ads, and declines in traditional forms of digital advertising. Publishers have cited the First Amendment and rejected restrictions on native advertising.
The guide makes clear that potential FTC liability extends to “everyone who participates directly or indirectly in creating or presenting native ads.” In 2016, look for the FTC to bring enforcement actions that highlight specific issues in the policy statement and guide, says Ervin. “For years, the FTC has been expressing concern that native advertising is misleading. They’re not going to go to the trouble of issuing guidance and then not do anything with it.”
[PDF Download: 2016
| |
[Web Index: 2016 Regulatory
|
Contacts
Insights
Publication | 03.01.26
Publication | 02.19.26
The QICDRC Practice Direction on the Use of Artificial Intelligence
Publication | 02.06.26



