Food & Beverage — The Belt Gets Tighter
Publication | 01.19.16
Food and beverage companies face heightened legal scrutiny in 2016, as the first in a series of sweeping new Food and Drug Administration (FDA) safety rules begin to take effect and battles over food labeling requirements continue.

John Fuson and Andrew Kaplan.
Last fall, the FDA began issuing new rules developed under the landmark Food Safety Modernization Act of 2011, and the rollout is expected to continue into the spring. The first compliance deadlines, which apply only to the largest food companies, come up this August. The rules usher in a new era in food safety regulation in which the FDA doesn’t have to wait for a food-borne illness outbreak to detain food it believes is adulterated. The FDA is now empowered to do so if a manufacturer simply doesn’t have a required food safety plan. “Food manufacturers now have the burden of showing they have adequate controls in place,” says John Fuson, a Crowell & Moring partner who served as associate chief counsel at the FDA from 2007 to 2012.
As the compliance deadline passes, Fuson expects to see immediate enforcement action in the form of warning letters from the FDA. Food manufacturers can look to the seafood and juice industries—which have long been required to have food safety plans—to see what type of scrutiny to expect.
Crowell & Moring partner Andrew Kaplan says the flood of new regulation comes at a time when food companies face growing civil and criminal penalties for mishandling foodborne illness outbreaks. In one extreme case last year, a peanut company executive was prosecuted by the Department of Justice (DOJ) and sentenced to 28 years in prison for his role in a salmonella outbreak in 2008 and 2009 in which nine people died. “Food manufacturers are in a tough enforcement environment where they are getting hit from all sides,” says Kaplan, who served as an attorney for the DOJ from 2003 to 2008.
Fuson says there may be some industry pushback against the new regulations on protectionism and restraint-of-trade grounds, particularly those regulations requiring food importers to verify the safety practices of their foreign suppliers.
LABELING BATTLES
Last year, after a handful of states passed genetically modified organism (GMO) labeling laws, the battle over whether food manufacturers should have to disclose that their products include GMOs moved to Congress. The House of Representatives swiftly passed a bill that would bar states from enacting mandatory GMO labeling, but it was unclear if the measure had the necessary support in the Senate. Meanwhile, the FDA seems finally ready to wade into debates over the meaning of words like “natural.”
Nonetheless, while the pace of class action litigation over GMO labeling seems to be slowing, as manufacturers have gotten savvier about using label wording, Kaplan sees new threats emerging. For example, four beverage makers that adopted label language touting their manufacturing process were hit with potential class action lawsuits in 2015 by plaintiffs claiming they were misled by buzzwords.
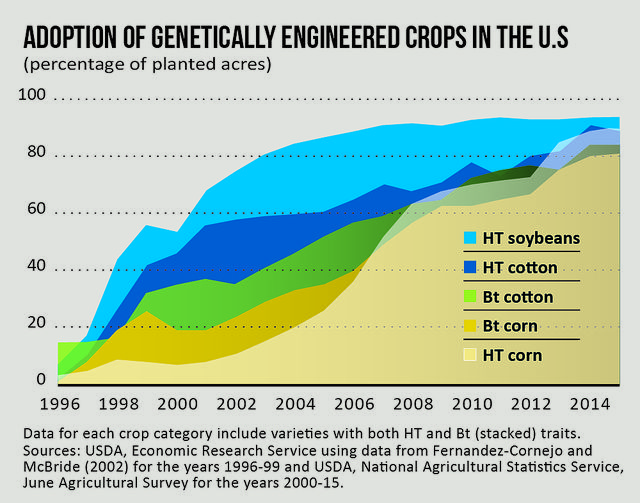
[The percentage of U.S. farmland planted with genetically engineered crops has surged in the past 20 years, and battles over how to label foods containing GMOs are being waged in both Congress and state legislatures across the country.]
[PDF Download: 2016
| |
[Web Index: 2016 Regulatory
|
Contacts
Insights
Publication | 03.01.26
Publication | 02.19.26
The QICDRC Practice Direction on the Use of Artificial Intelligence
Publication | 02.06.26



