FDA Enforcement Trends: Reflecting on 2019 and Looking Onward to 2020
Client Alert | 16 min read | 01.16.20
Inspections
While the number of enforcement actions implemented by the Food and Drug Administration (FDA) has decreased throughout the years – the quantitative data tells only part of the story. A review of this past year’s enforcement actions reveal that the agency remains committed to enforcing compliance with its regulations by utilizing a risk-based approach to advance its public health priorities and minimize public health risk.
This article is the first installment of a four-part series which leverages available FDA enforcement data from 2016 to present, with an emphasis on the pharmaceutical and medical device industries, to provide companies with insight on how to best comply with FDA regulations and avoid common pitfalls in 2020. This series will focus on trends in FDA (1) inspections; (2) Form 483s; (3) warning letters; and (4) seizures and injunctions.
FDA Enforcement Tool Belt
The FDA has a variety of enforcement tools designed to encourage and compel compliance with regulations. These tools include: Form 483s, warning letters, seizures, injunctions and criminal prosecution and fines. This menu of enforcement options progress from least severe to most severe in degree of enforcement, as set out in the graphic below:
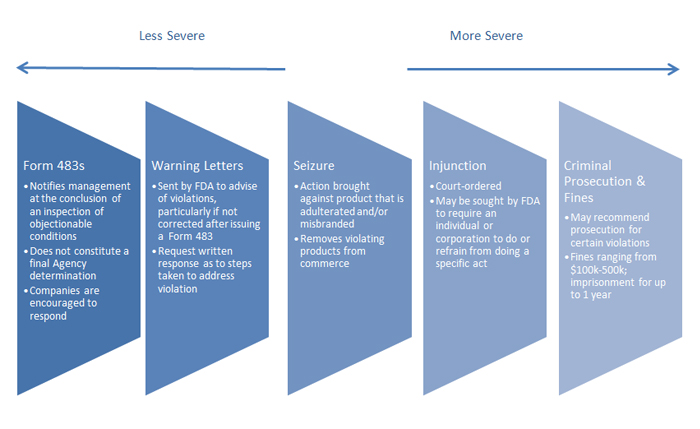
FDA enforcement actions can have a significant impact on a company’s reputation and relationship with the agency. In addition, we find that more and more often these enforcement actions are being used as tools by the plaintiffs’ bar to support their products liability and false advertising lawsuits. As such, analyzing FDA enforcement data is critical. Not only does it provide a snapshot of where the agency has been, but oftentimes this information reveals insight into future litigation risks for FDA-regulated industries.
Looking Back: Inspection Data
Inspections, while not necessarily FDA enforcement actions in and of themselves, are often a catalyst for enforcement actions. FDA uses inspections to gather information on a facility’s regulatory compliance. During an inspection, FDA inspectors may collect samples, make observations, and gather information about the operations of the facility and are conducted without notice, except at medical device facilities and foreign facilities. The FDA typically takes a risk-based approach for developing its inspection schedule. Drug and medical device manufacturing facilities are inspected every two years and low-risk facilities are typically inspected every two to five years. High-risk facilities (e.g. facilities with a history of non-compliance) are inspected at least yearly.
Table 1. Inspections of Registered Domestic and Foreign Drug and Device Establishments1
Year |
Domestic Inspections |
Foreign Inspections |
Total |
|
FY 2016 |
3,314 |
1,632 |
4,946 |
|
FY 2017 |
3,310 |
1,609 |
4,919 |
|
FY 2018 |
3,100 |
1,598 |
4,698 |
|
FY 20192 |
2,432 |
1,213 |
3,645 |
Table 1 demonstrates the steady decline in the number of inspections of domestic and foreign drug and device establishments from 2016 – 2019. As it pertains to domestic inspections, in 2016 and 2017, the number of inspections were nearly identical. However, in 2018 there were 210 fewer domestic inspections amounting to a drop of approximately 6.6%. While the final number of FDA inspections are not currently available for 2019, when this information is published we expect it will show a further drop in the number of inspections last year given that as of September 2019 the agency had conducted only 2,434 domestic inspections.
Similarly, there has been a consistent decline in the number of foreign FDA inspections of drug and medical device facilities from FY 2016 – 2018, and the number of inspections are on track to further drop in FY 2019.
Looking Ahead to 2020: Inspection Tips
While FDA inspections can lead to enforcement actions, they are also an opportunity for companies to develop a dialogue with FDA inspectors, and build agency confidence in their manufacturing facility. Companies should view FDA inspections as motivation to comply with the Food Drug and Cosmetics Act and ward off potentially damaging enforcement actions, including Form 483 observations.
As we reflect on 2019, we have a few inspection tips FDA –regulated companies should keep in mind to ensure that they are inspection ready in 2020:
- Investigations must be thorough and complete. An all-to-common observation during an inspection is of investigations into deviations that are not closed in a timely manner, are not complete in their evaluations of operations or product on the market, or that have not determined root cause. Make investigations a priority to ensure that they are current and complete before FDA investigators arrive.
- Adverse events must be reported. FDA relies on adverse event reports to monitor concerns across product categories. If data isn’t reported, the agency cannot accurately identify emerging risks and timely respond to problems. The agency therefore reacts aggressively when adverse event information is withheld or reported slowly. Making sure that adverse event reports are thorough and robust will help set a good tone with investigators during an inspection.
- All personnel must be trained appropriately for their tasks. Investigators will want to talk to line operators during an inspection, to know what they are doing and how well they are trained for their jobs. Making sure that personnel are well trained will give them confidence to respond to investigator questions.
- Inspection observations must be responded to promptly and fully. Inspection observations and Form 483s happen. When they do, it’s important to respond promptly and fully, to make sure the agency concerns are addressed. Take a comprehensive approach to responses to make sure they speak to a broad audience who may not be familiar with the facility, not just the on-site investigators.
- Quality Unit staffing and resources must be appropriate for operations. Inherent to all of the above is ensuring that the Quality Unit has the personnel and resources needed to fully meet the demands of operations at a facility.
Conclusion
FDA’s inspections have been on the decline however, in the words of former FDA Commissioner Scott Gottlieb: “It’s not the number of inspections we do, it’s whether we’re targeting effectively.”
As such, it’s important to peel back the layers to better understand FDA’s enforcement priorities. One way to assess FDA’s areas of focus is by analyzing FDA Form 483s (Notices of Inspectional Observations). Form 483s are good indicators of what the FDA is prioritizing during its inspections and assists with answering important questions such as: What were the top 483 observations made during FDA inspections in 2019? How does this data compare to prior years? Which FDA-regulated facilities received the most 483 Observations? FDA Form 483 observations will be the subject of Part 2 in this four-part series.
1 These inspections consist of CGMP inspections for drugs and QS inspections for Class II and Class III devices.
2 Data available through September 2019.
Contacts
Insights
Client Alert | 4 min read | 03.05.26
The U.S. Department of Labor (DOL) has proposed another revision to independent contractor regulations, one that would provide for more leeway in classifying workers as contractors. DOL’s proposed rule, published on February 26, 2026, would rescind the Biden DOL’s March 2024 independent contractor regulation and reinstate a framework substantially tracking the prior Trump rule of January 2021. The proposed rule would also apply the narrower analysis to worker classifications under the Family and Medical Leave Act (FMLA) and the Migrant and Seasonal Agricultural Worker Protection Act (MSPA). The comment period closes in late April 2026; until then, the 2024 rule remains in effect for purposes of private litigation.
Client Alert | 8 min read | 03.05.26
Client Alert | 4 min read | 03.04.26
Sixth Circuit Finds EFAA Arbitration Bar to Entire Case — Not Just Sexual Harassment Claims
Client Alert | 3 min read | 03.02.26



